Non-Cigarette Tobacco Products and POS Policies
What are Non-Cigarette Tobacco Products?
With restrictions on the sales and marketing of cigarettes, tobacco companies have expanded and promoted non-cigarette tobacco products (sometimes called “other tobacco products” or OTP). These products also may have strong youth appeal, offering milder-tasting and sweet flavored varieties of tobacco. While all tobacco and nicotine products fall under the Food and Drug Administration (FDA)’s regulatory authority, not all are regulated the same. For example, while flavors other than menthol in cigarettes have been prohibited since 2009, the only other products with any flavor restrictions are cartridge-based e-cigarettes, which can now only be sold in tobacco and menthol flavors.
Non-cigarette tobacco products include, but are not limited to:
Cigars, cigarillos, and little cigars. The FDA defines a cigar as “a roll of tobacco wrapped in leaf tobacco or in a substance that contains tobacco.” However, there are several different types of cigars, some of which (“little cigars” or “filtered cigars”) are virtually indistinguishable from cigarettes.[1] Many are sold for less than $1 and come in sweet and fruity flavors that appeal to youth. Learn more in the Campaign for Tobacco-Free Kids’ report Not your Grandfather’s Cigar and the Truth Initiative’s Cigars: Facts, stats, and regulations.
Smokeless tobacco (e.g. snus, chew, dip, snuff). These products are not smoked but places between the gum and the cheek or lip. See the Truth Initiative’s The truth about: smokeless tobacco.
Oral nicotine pouches. This a newer sub-category of products is similar to the smokeless, spitless tobacco product snus, but the pre-filled pouches, which are designed to be placed between a user’s lip and gum contain nicotine powder rather than tobacco leaf. They are often advertised as “tobacco free” though they usually contain nicotine derived from tobacco. Some claim to use synthetic nicotine. Popular brands include Zyn, On!, Velo, and Rogue. They also come in a variety of flavors that appeal to youth as well as a variety of nicotine levels. For more information, see our podcast episode on oral nicotine pouches and other “modern” oral nicotine products. Truth Initiative’s report Zyn and other oral nicotine pouches.
Dissolvables (e.g. orbs, strips, sticks). Nicotine products that dissolve in the mouth and do not require spitting, but resemble products like mints, mouthwash strips, and toothpicks and come in a variety of flavors.
Roll-your-own and pipe tobacco. Loose tobacco sold to be either rolled into cigarettes or burned in a pipe. For more information see the Campaign for Tobacco Free Kids’ factsheet on “The Problem with Roll-Your Own and Other Smoking Tobacco.”
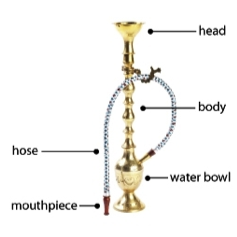
Hookah. Tobacco smoked through a waterpipe and also known as shisha, argileh, narghile, and maasel). Learn more in our evidence summary on hookah tobacco at the point of sale.
Electronic cigarettes. Products that heat a liquid solution containing nicotine, flavoring, and other chemicals into an aerosol that is inhaled by the user. They are also called vapes, vape pens, vaporizers, e-hookahs, and electronic nicotine delivery systems (ENDS). Learn more in our evidence summary on e-cigarettes at the point of sale.
Heated tobacco products. According to the CDC, “heated tobacco products heat processed tobacco leaf, allowing users to inhale nicotine into their lungs.” They are sometimes also called “heat-not-burn” products and vary in design. iQOS is an example of a heated tobacco product that is currently expanding in the US market. Learn more in our evidence summary on heated tobacco products at the point of sale.
See descriptions of each products from the FDA here.
For young people, these mild and often sweet product varieties may serve as the entry to trying more potent products as they acquire a taste for stronger tobacco. E-cigarettes have been the most commonly used tobacco product among youth since 2014, and current use of more than one type of tobacco product is also on the rise, with 8.2% of high school students reporting current use of 2 or more tobacco products in 2020.[2] Further, communities that allow sales of such products near schools show higher rates of youth tobacco use.
Smokeless products may additionally entice and enable smokers to continue to use tobacco, even if driven to quit smoking because of social stigma, harm to their families, or the inconvenience of not being able to smoke in public places.
The marketing for these products are also not evenly distributed. Smokeless tobacco ads are more prevalent in rural areas and neighborhoods with more white residents, while cheap, flavored little cigars and cigarillos ads are more common in lower income neighborhoods and neighborhoods with more Black residents. [3, 4, 5]
Campaign for Change
These non-cigarette tobacco products are also harmful and addictive. They contain a range of known carcinogens, and have been shown to cause cancers and a range of other oral health conditions and other chronic diseases. In addition to starting a long-term nicotine addiction, early use of tobacco has short-term health effects too, including respiratory and cardiovascular impairment in adolescence and young adulthood.
“There is no such thing as safe tobacco” stated the slogan throughout the Delaware Tobacco Prevention and Control Program’s The Dirty Truth campaign. This campaign has generated powerful communication materials, including animated banner ads for the web, gas pump tops, bus interior signs, radio spots, and other compelling materials. It now focuses on vaping.


Modified Risk Tobacco Products
The modified risk tobacco product (MRTP) pathway is “outlined in the 2009 Family Smoking prevention and Tobacco Control Act [and] allows companies to submit applications for the FDA to evaluate whether a tobacco product may be sold or distributed for use to reduce harm or the risk of tobacco-related disease associated with commercially marketed tobacco products.” The FDA maintains that the products authorized to be marketed with this claim are neither safe nor FDA-approved.
General Snus: In 2019, for the first time, the FDA authorized a tobacco product to be marketed with a ‘modified risk’ claim. Granted to Swedish Match USA, Inc., eight snus smokeless tobacco products, sold under their ‘General’ brand name, can be marketed with the claim that “using General Snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.” Packaging and advertising for these products must continue to include the warning statements required for all smokeless tobacco products.
iQOS: In July 2020, the FDA authorized Philip Morris to market its IQOS Tobacco Heating System as a ‘modified risk tobacco product’, making the IQOS device and its accompanying HeatSticks only the second set of products to be authorized as MRTP and the first tobacco products to receive “exposure modification” orders. With this authorization, Philip Morris can now market claims that the IQOS system “heats tobacco but does not burn it” and that completely switching to the product from cigarettes “significantly reduces the production of harmful and potentially harmful chemicals”; they can also advertise that “scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful products.” Whether these products are truly safer than cigarettes remains questionable, with more research needed on both the short- and long-term effects on morbidity and mortality. In 2018 FDA Tobacco Product Scientific Advisory Committee found that the IQOS did not substantially reduce risks of tobacco-related death and disease, and in 2019, FDA did not approve the products for a “risk modification” label. The nicotine in the product is highly addictive, and independent, peer-reviewed research assessing the short- and long-term effects of these products is severely lacking. As well, a significant portion of the current research has been led, funded by, or in some way associated with the tobacco industry. As a result, the authorization requires both the FDA and Philip Morris to continue to monitor the appropriateness of the modified risk order and its impact on youth use. In response to the FDA authorization, multiple organizations, including the Campaign for Tobacco-Free Kids, American Cancer Society Cancer Action Network, American Heart Association, American Lung Association and Truth Initiative, issued a joint counterstatement, arguing the authorization puts youth and the public’s health at risk. Their concerns center on Philip Morris International’s marketing of IQOS as a safe, sleek, and stylish product, in a similar way that e-cigarettes were marketed, and concerns that this will lead to non-smokers and youth, in particular, being enticed by the advertising and initiating tobacco use.
A Smokefree Future?
While Big Tobacco companies like Philip Morris International claim to be working towards a smoke-free world and transitioning away from cigarettes, they spent over $8.4 billion in 2018 alone marketing and promoting conventional combustible cigarettes. While they are introducing new categories of products (e.g. heated tobacco products, oral nicotine pouches and lozenges) that are not combusted, they are still aggressively promoting cigarettes, the known leading cause of preventable death and disease, both in the U.S. and around the world and continuing to introduce new cigarette and cigar products as well (especially in low- and middle-income countries). Learn more about the industry’s attempts to appear like they are in favor of harm reduction in Truth Initiative’s report on Spinning a New Tobacco Industry: How Big Tobacco is Trying to Sell a Do-Gooder Image and What Americans Think About it and see additional information about the Philip Morris-funded Foundation for a Smoke-Free World from the Campaign for Tobacco Free Kids.
POS Policy Opportunities
Consider policy options to protect the health of your community:
- Sales restrictions on flavored tobacco products: In a growing movement, localities and states around the country are extending the federal ban on the sale of flavored cigarettes to include menthol and all flavored tobacco products. New York City was the first place to successfully ban flavored non-cigarette tobacco products, and defended this policy in court. In a similar fashion, the state of Maine banned of sales of flavored cigars, and Providence, Rhode Island also prohibited flavored non-cigarette tobacco products. Since then, places like Minneapolis have restricted the sales of all flavored tobacco products to adult-only specialty tobacco stores, and places like Chicago have prohibited the sale of flavored tobacco, including menthol, near schools. San Francisco was the first city to prohibit the sale of all flavored tobacco products without exception, including menthol cigarettes and non-tobacco flavored e-cigarettes, and additional communities around the country are following suit. As of February 2021, over 300 U.S. jurisdictions had some type of restriction on flavored tobacco product sales and the list continues to grow.
- Increasing prices of non-cigarette tobacco products: Price increases can dissuade consumers, and young consumers in particular, from tobacco products. Increases in price can come from excise taxes and from non-tax approaches like minimum floor prices and prohibiting the redemption of coupons and discounts. Minimum prices for all tobacco products can help discourage people from switching to another harmful tobacco product when prices are raised on cigarettes. See policy solutions of non-tax approaches to raising product prices.
- Licensing of retailers: Licensing retailers for all tobacco products, including cigarettes, e-cigarettes, and all other ‘OTP’ offers a range of opportunities to monitor and enforce point-of-sale policies, collect licensing fees, or restrict retailer presence, for example, near schools. It may be important to ensure that the definition of tobacco in your jurisdiction’s laws is comprehensive enough to cover all types of nicotine and tobacco products. Learn about other policy options for licensing retailers to see what may work best for your community.
- POS marketing, advertising and promotional restrictions: While federal law places limits on local and state governments’ abilities to regulate the content of cigarette marketing or promotions, non-cigarette tobacco products may be regulated at the point of sale, with appropriate policy language that does not raise First Amendment concerns. See other policy opportunities for limiting marketing, advertising, and promotions at the point of sale.
- POS health warnings, social marketing campaigns, and other communication materials: Graphic warnings at the point of sale and campaign materials, such as The Dirty Truth’s pump tops for gas stations with convenience stores, could educate consumers about the risks of using non-cigarette tobacco products. Media campaigns addressing emerging products may be important to counter perceptions of reduced risk that are associated with flavors and that may be conferred with “modified exposure” marketing claims. See other policy options, including voluntary programs, that can be used to promote health warnings at the point of sale.
Overall, regulation of non-cigarette tobacco products is critical to keep up with the changing product offerings developed and promoted by tobacco companies. Such policies can promote local business interests as well as prevent young people from having easy access to flavored varieties marketed directly to them.
Resources
- FDA Tobacco Products, Ingredients & Components
- CDC’s E-Cigarette, or Vaping, Products Visual Dictionary
- Campaign for Tobacco-Free Kids
- Fact sheets from the Truth Initiative:
- CounterTobacco.org’s evidence summaries:
- From the Public Health Law Center:
- Surgeon General’s Report: Preventing Tobacco Use Among Youth and Young Adults booklet and full report
Next Steps
- Familiarize yourself with policy options
- Conduct store assessments to see what products exist in your community
- See Sample Language to Restrict the Sale of Flavored Non-cigarette Tobacco Products, Including Menthol from the Public Health Law Center
- Read about New York City court case and successful defense of banning flavored non-cigarette tobacco products
- See policy options for Regulating Flavored Tobacco Products from the Public Health Law Center